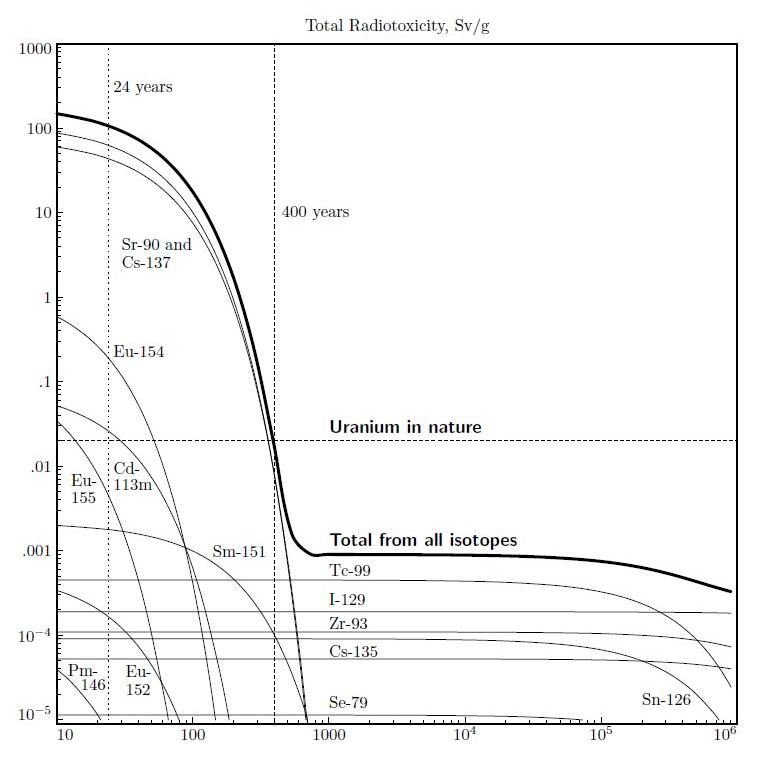

The radiotoxicity of uranium in nature was taken from Återanvändning av långlivat kärnavfall och sluten bränslecykel möjlig i nya reaktortyper (Recycling of long-lived nuclear waste and a closed fuel cycle are possible in new reactor types), Nucleus 4 (April 2007), page 15, [in Swedish], by Janne Wallenius.
Yttrium-90 (90Y) does not appear. It is not a long-lived fission product. Its half life is 2.671 days. It persists as a long-lived source of radiotoxicity because it is the decay product of 90Sr, which has a half life of 28.79 years. The relationship between them is called secular equilibrium. The dose factors in ICRP 119 include the effect of daughter isotopes. Once yttrium is separated from strontium, 90Y decays to stable 90Zr, and the remaining yttrium is effectively non-radioactive within 30 days.
Ideally, strontium and caesium should be separated from other fission products. There's a newer proposal to cleanse electrolyte that is probably easier. Together, all their isotopes constitute 9.23% of all fission products, but they account for 99.39% of radiotoxicity.
Yucca mountain was proposed as a repository for un-processed spent fuel. Pretending that materials that are that dangerously radiotoxic can be hidden from humanity for 300,000 years is daft. The pyramids were plundered before 500 years!
The Waste Isolation Pilot Project (WIPP) is evaluating the possibility to use geological storage for un-processed spent fuel.
Charles S. Forsberg has a better idea how to do geologic storage.
Spent fuel should be processed to separate unused fuel from fission products. Unused fuel should be returned to reactors to make electricity and fission products.
Instead of geological storage on land, another possibility is disposal at sea using impervious insoluble ceramics. Concrete can be very stable in seawater. There are intact dockworks at Caesarea, in Israel, that were poured by Roman engineers 2,000 years ago. After 2,000 years, modern chemists have finally worked out the Roman cement formula.
The entire assembly can then be dropped into the Marianas Trench, or into the Pacific Ocean halfway between Alaska and Hawaii. It will sink several hundred feet into the mud at the bottom. The stability of the concrete dockworks poured by Roman engineers more than 2,000 years ago at Caesarea demonstrates that seawater will not reach the impervious insoluble ceramic before 3,000 years. That's 100 half lives of strontium and caesium.
One mole of any substance is a mass in grams equal to its atomic weight -- about 90 grams of strontium-90. One mole of any substance contains 6.023 x 1023 atoms (or molecules, if a compound). This is called Avogadro's number. If you divide that by two, 100 times, the result is 4.75 x 10-7. This shows that after 3,000 years — 100 half lives — there is one chance in two million that one radioactive atom remains from those original 90 grams.
Others have very short half lives, but are in secular equilibrium with longer-lived isotopes. For example, yttrium-90 (90Y), which has a half-life of 2.671 days, is the decay product of strontium-90 (90Sr), which has a half life of 28.79 years. Antimony-126n (126nSb) is a decay product of tin-126 (126Sn), which has a half life of 230,000 years. 126nSb decays with a half life of eleven seconds to 126mSb, which decays with a half life of 19.1 minutes, to 126Sb, which has a half life of 12.4 days, and decays to stable tellurium-126 (126Te). 126nSb, 126mSb, and 126Sb are in secular equilibrium with 126Sn.
Other fission products have extremely long half lives. For example, zirconium-94 (94Zr) has a half life of 6.0 quadrillion years -- 435 million times the age of the universe. Extremely long half life means extremely low activity (they are inversely proportional), which means extremely low radiotoxicity. IRCP publication 119 does not provide dose factors to convert radioactivity to radiotoxocity, for isotopes with half lives longer than one billion years, or shorter than ten minutes.
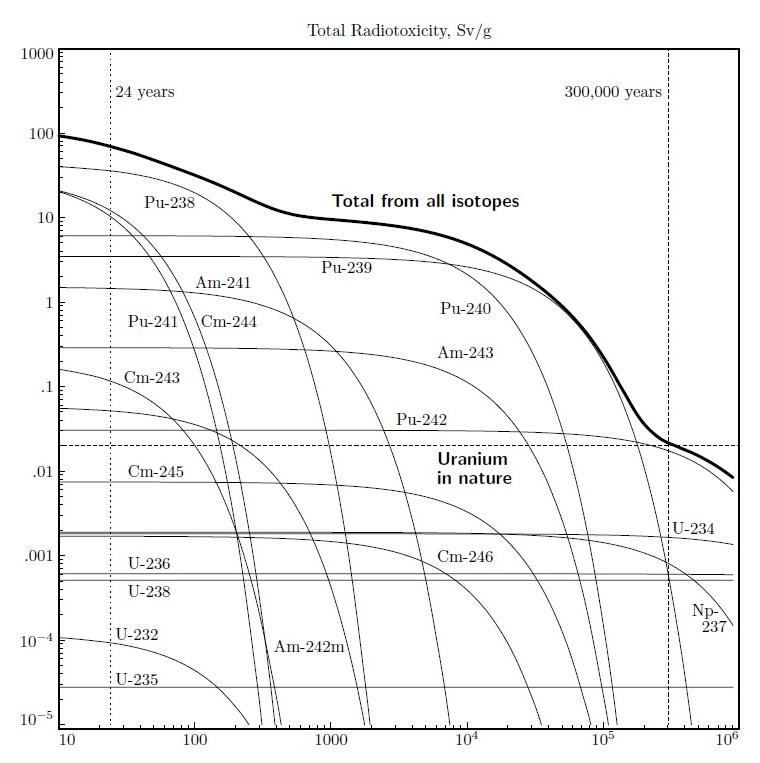
Radiotoxicity curves for all actinides in unused fuel with half lives greater than ten years show why it is important to separate fission products from unused fuel.
Directly fissionable isotopes such as 235U and 239Pu
can be consumed in light-water reactors. Some isotopes, such as
238U, can be transmuted in light-water reactors by neutron
absorption (albeit not efficiently), and radioactive decay, to fissionable
isotopes, and then consumed. For example
238U + n → 239U → 239Np +
β → 239Pu + β
Non-fissionable isotopes that are not transmuted, or inefficiently
transmuted, and therefore not consumed, simply accumulate, even if fuel is
recycled. These isotopes can, however, be efficiently transmuted, and
then consumed, in fast-neutron reactors such as the Integral Fast
Reactor.